The Science Behind TB006
Background
What is TB006?
How It Works
How does TB006 work?

The Science
What is the science behind TB006?
The following results come from both self-reported progress and clinical reporting from providers.
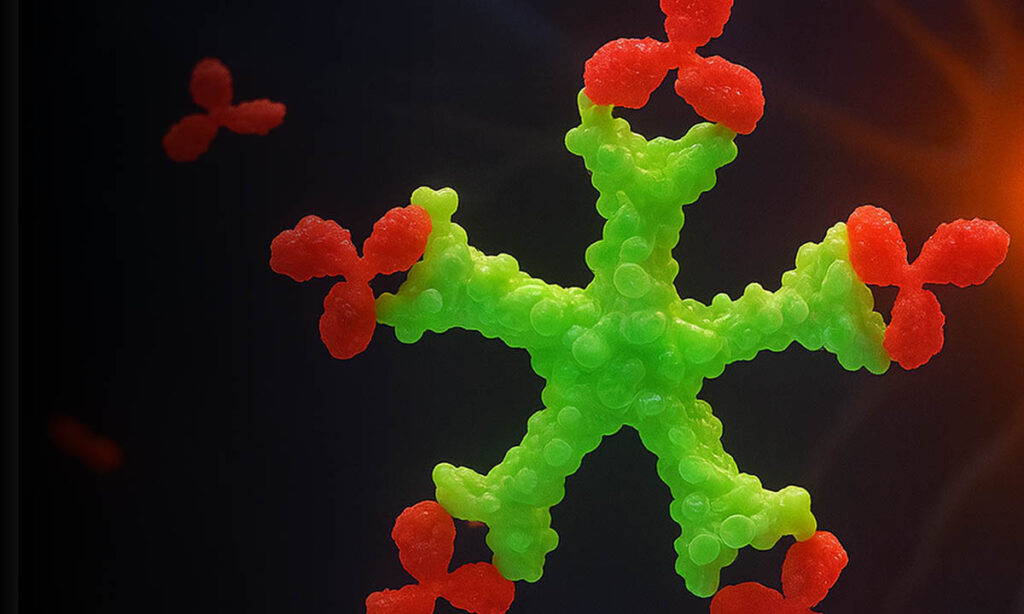
The Core Issues
GAL-3 has many functions.
Its first function is to be a first responder, calling on the immune system to rescue damaged cells. In the right amounts, GAL-3 aides in healing. In addition, it frequently gets overproduced and creates a negative feedback loop associated with many chronic diseases.
Excess GAL-3 in the brain disrupts the immune system, rendering the immune system partially to totally dysfunctional, damaging our brain, and causing dementia of all types and severities.
In scientific terms, GAL-3 contributes to forming toxic oligomers and plaques, disrupting communication between neurons and leading to cognitive decline. GAL-3 overactivation triggers excessive microglial activity and chronic neuroinflammation, further damaging neurons.
TB006 neutralizes GAL-3 and allows the immune system to revitalize and heal the brain, thereby enabling the promotion of improved levels of cognition and other brain functions for individuals.
The Core Issues
What dementias could be treated with TB006?
Early clinical trials show promising results for treating various dementias, including:
Vascular dementia
Frontotemporal dementia
Lewy Body dementia
Dementia with mixed pathology
Comparison
How does TB006 compare to other FDA-approved Alzheimer’s drugs?
While there are FDA-approved drugs on the market for the treatment of Alzheimer’s Disease, these drugs are only able to slow the progression of Alzheimer’s Disease, and come with serious unwanted side effects.
Specifically, leading Alzheimer’s drugs, Lequimbi and Kisunla, all show signs of disease slowing after 18 months, but none show signs of disease reversal like TB006. And all three drugs showed ARIA side effects at greater rate than would be expected in normal aging Alzheimer’s patients, compared to no ARIA side effects in TB006 patients.
ARIA = Amyloid Related Imaging Abnormalities. Brain bleeds and brain swelling seen on Brain CT or Brain MRI. Monthly imaging is required for Lequimbi and Kisunla, but not for TB006.

Side Effects
What are the side effects of TB006?
Thus far, TB006 has demonstrated to be safe. TB006 is very selective. Very few medications are contraindicated and its singular selectivity permits it to avoid metabolic interaction. This significantly contributes to its high safety profile. TB006 is administered by an IV drip. In a study of 72 people, only 4 people reported minimal side-effects.
The side effects were:
- Swelling at the site of the IV
- Feeling light headed after the IV.
These are common side-effects for any IV administration.
How to get access to TB006?
Patients must qualify for the expanded access program and meet certain requirements. We may be able to help you find a physician offering this program.
